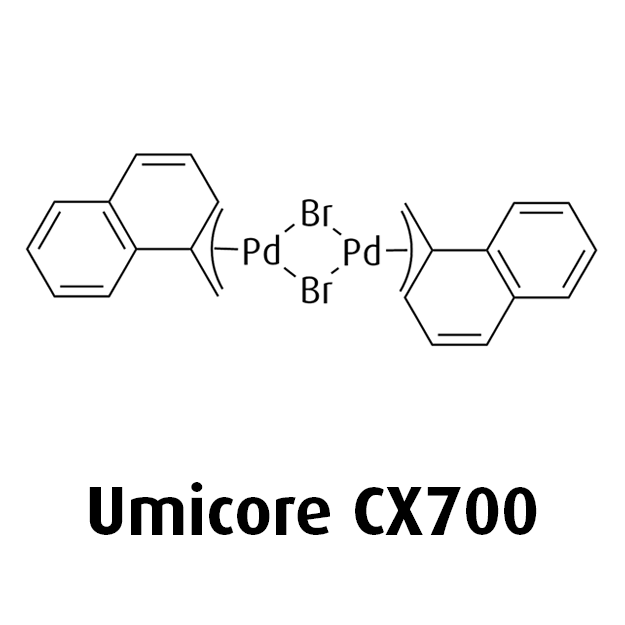
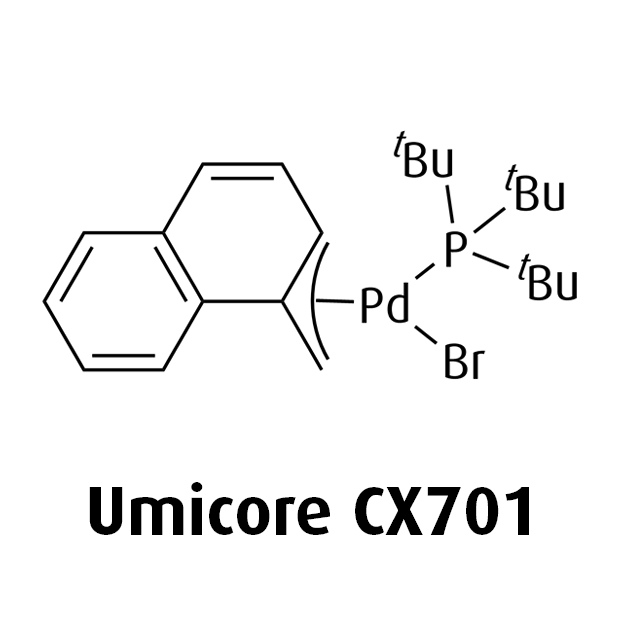
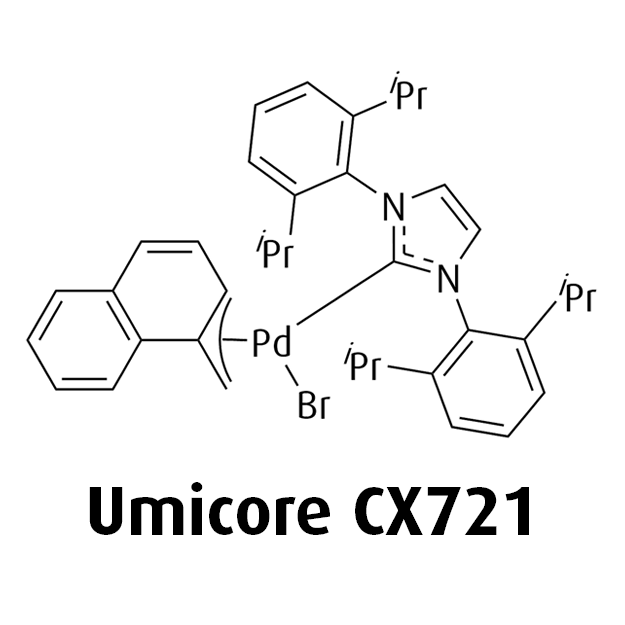
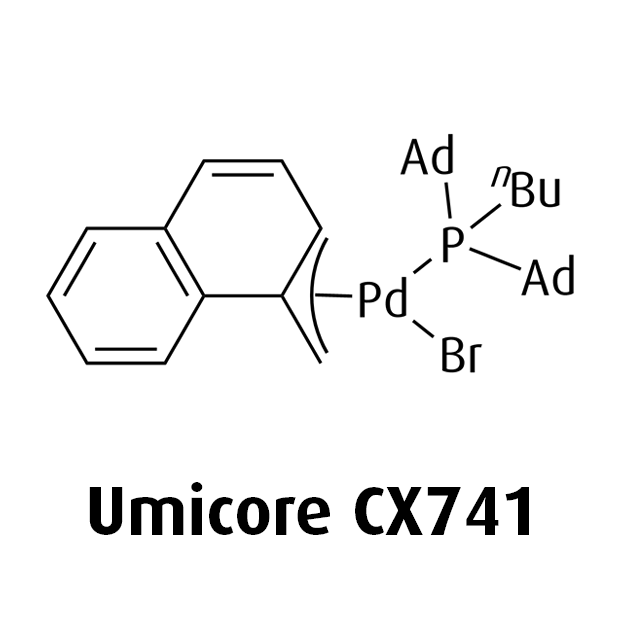
MeNAP CX catalysts
Highly effective industrial C-C / C-N cross-coupling catalysts
At Umicore PMC, we harness state-of-the-art catalysts such as MeNAP CX to revolutionize cross-coupling reactions. MeNAP, also known as Di(µ-bromo)bis(1-methylnaphthyl)dipalladium(II), acts as an exceptionally fast activating precatalyst, that works seamlessly with preferred ligands to enable very specific and selective coupling reactions
Key Features of the MeNAP CX Catalysts:
- Specialized in executing challenging cross-coupling reactions like Suzuki-Miyaura couplings
- Able to facilitate the formation of tetra-ortho-substituted biaryls at room temperature
- Enables challenging reactions with low catalyst loading, enhancing efficiency
- Offers flexibility by combining with various phosphine, NHC, or Buchwald ligands
- Allows fine-tuning for individual reactions, expanding the range of accessible substrates
- Enhances substrate scope by adapting to different ligands, broadening applicability
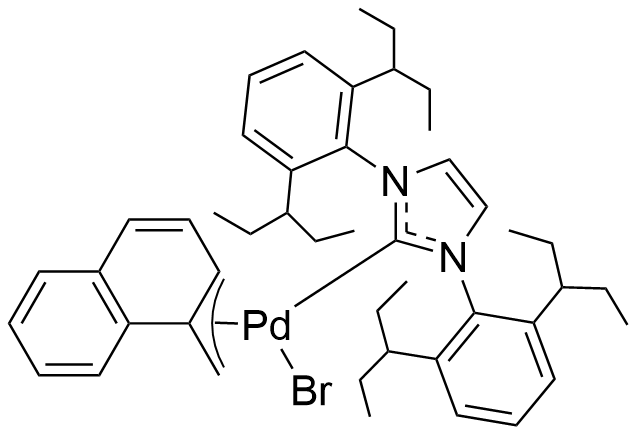
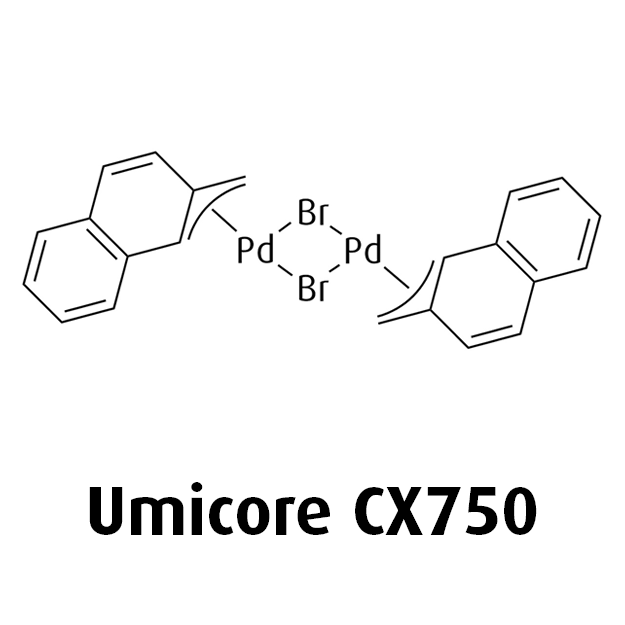
Umicore CX724
1-Methylnaphthyl[1,3-bis(2,6-Di-3-pentylphenyl)imidazol-2-ylidene]bromopalladium(II)
Empirical formula: [(IPent)Pd(α-MeNAP)Br]
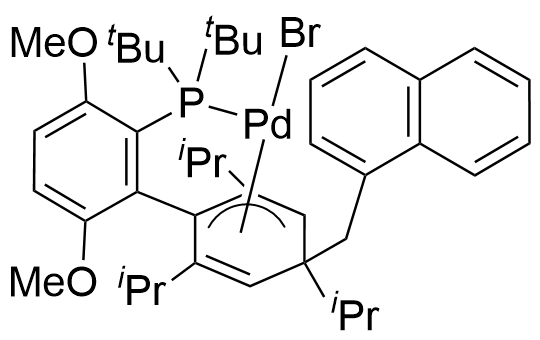
Umicore CX737
1-Methylnaphthyl[3,6-dimethoxy-2',4',6'-tris(1-methylethyl)[1,1'-biphenyl]-2-yl]bis(1,1-dimethylethyl)phosphine]bromopalladium(II)
Empirical formula: Pd(α-MeNAP)(tBuBrettPhos)Br
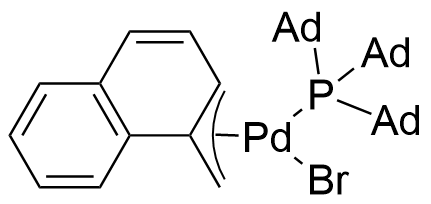
Umicore CX742
1-Methylnaphthyl[tris(1-adamantyl)phosphine]bromopalladium(II)
Empirical formula: Pd(α-MeNAP)(PAd3)Br
B. Xue, F. Papp, M. Yang, J. Shen, A. Doppiu, L. J Gooßen, ACS Catal., 2024, 14, 15, 11172–11177: Pd-Methylnaphthyl-tBuBrettPhos Complexes as Efficient and Selective Catalysts for the Monoarylation of Ammonia and Hydrazine https://pubs.acs.org/doi/10.1021/acscatal.4c02624
D. Sowa Prendes, F. Papp, N. Sankaran, N. Sivendran, F. Beyer, C. Merten, L. J. Gooßen, Angew. Chem. 2023, e202309868: Enantioselektive Synthese von Arylglycinen durch Pd-katalysierte Kupplung von Schöllkopf-Bislactimethern mit Arylchloriden https://doi.org/10.1002/ange.202309868
Angew. Chem. Int. Ed. 2023, e202309868: Enantioselective Synthesis of Arylglycines via Pd-Catalyzed Coupling of Schöllkopf Bis-Lactim Ethers with Aryl Chlorides https://doi.org/10.1002/anie.202309868
Palladium-Catalyzed γ-Arylation of Acylketene Synthons with Aryl Chlorides Enabled by Ylide-Functionalized Phosphines (YPhos). - Manna, S., Papp, F., Hisata, Y., Löffler, J., Rybka, M., Gessner, V.H., Hoshimoto, Y. and Gooßen, L.J. - Adv. Synth. Catal. 2024
https://doi.org/10.1002/adsc.202301474
Enantioselective Synthesis of Arylglycines via Pd-Catalyzed Coupling of Schöllkopf Bis-Lactim Ethers with Aryl Chlorides. - D. Sowa Prendes, F. Papp, N. Sankaran, N. Sivendran, F. Beyer, C. Merten, L. J. Gooßen - Angew. Chem. Int. Ed. 2023, e202309868.
https://doi.org/10.1002/anie.202309868
Selective Monoarylation of Ammonium Triflate with Aryl Chlorides Catalyzed by [Pd(β-MeNAP)Br]2 and AdBrettPhos - B. Xue, J. Shen, S. Manna, A. Doppiu, L. J. Goossen - Adv. Synth. Catal. 2023, 365, 20, 3473-3477.
https://doi.org/10.1002/adsc.202300761
Halogen-bridged Methylnaphthyl Palladium Dimers as Versatile Catalyst Precursors in Coupling Reactions - N. Sivendran, N. Pirkl, Z. Hu, A. Doppiu, L. J. Gooßen - Angew. Chem. Int. Ed. 2021, 68, 25151-25160.
https://doi.org/10.1002/anie.202110450