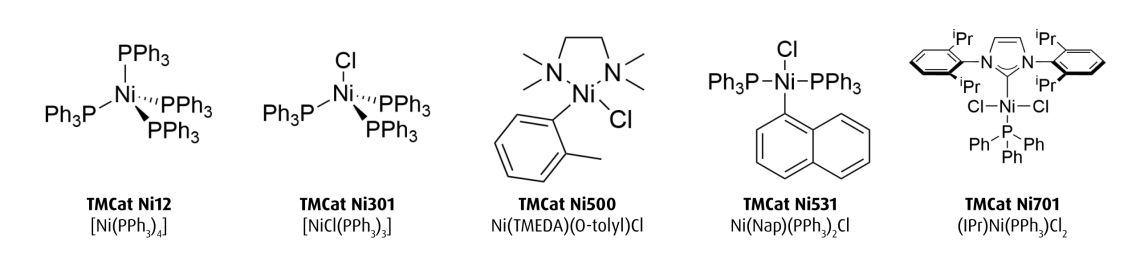
TMCat Ni - a novel base metal catalyst portfolio
Innovative homogeneous base metal catalysts: expanding the horizons of catalysis
Umicore PMC introduces a novel base metal catalysts product line offering additional reactivity and sufficient stability in chemical transformations facilitating applications in fine chemicals, pharmaceuticals and agrochemicals synthesis.
Homogeneous base metal catalysts are a promising addition to traditional platinum group metal (PGM) catalysts. While high activities have enabled an expanded scope of reaction partners, evolving ligand architectures have resulted in improved stability and efficiency. Though the scope of application overlaps with traditional PGM catalysts (fine chemicals, pharmaceuticals, and agrochemicals for example), base metal catalysts can offer unique reactivity trends resulting from alternative mechanistic pathways. Umicore PMC is committed to providing its customers with the highest quality products and services and differentiates itself by offering advanced, innovative solutions. We believe that our new line of homogeneous base metal catalysts will help us achieve this goal.
Umicore is partnering with key industrial end-users as well as academic experts to design the most comprehensive, customer-centric, non-PGM homogeneous catalyst portfolio available. This begins with a proactive set of Nickel cross-coupling catalysts but will extend to other metals and application areas in the future. As a customer-driven effort, catalyst selection will be largely based upon recommendations received from collaborations with industrial process scientists and leading figures in academia. We plan to focus on, but not limit ourselves, to products that can be readily used in manufacturing operations. Catalysts in the Umicore portfolio shall be available at multi-kg scale when required.
The initial offering includes the following catalysts:
Key features of our non-PGM catalysts
Compared with palladium-based catalysts, complexes of nickel demonstrate a variety of differing and complementary reactivity. Nickel undergoes more facile oxidative addition, can adopt a variety of oxidation states (0, + 1, + 2, + 3, + 4), is more able to access one-electron reduction/oxidation pathways, and is less likely to facilitate β-hydride elimination. These features allow for successful reactions with many cross-coupling partners that were not feasible with traditional PGM catalysts.
While process chemists are keen to implement new technologies that have a positive impact on overall economics, to determine the optimal approach it is essential to conduct a comprehensive analysis that includes economic, ecological, and sustainability factors, as the best solution is unique for each synthetic challenge. Unfortunately, non-PGM catalysts currently lack the advanced recovery and recycling processes available for PGM-based materials. Therefore, unless manufacturing occurs in regions without recycling capabilities, PGM recovery must be included in a thorough assessment.