Z-Selective and Stereoretentive Grubbs Catalyst® Technologies
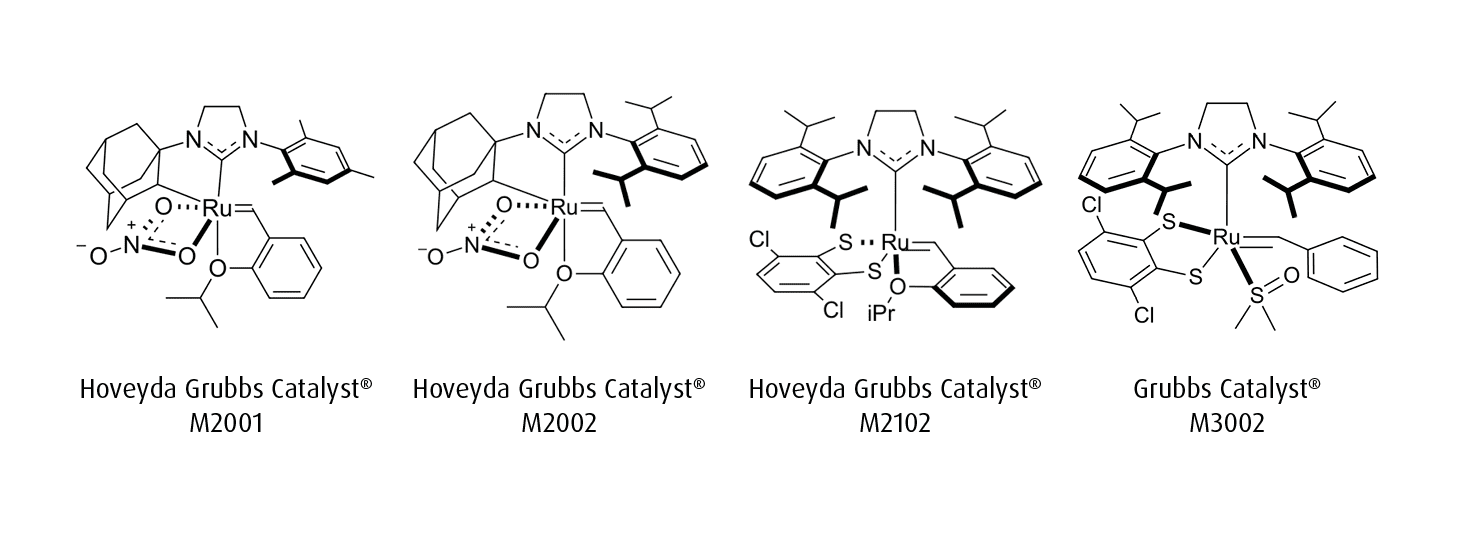
Z-selective and stereoretentive ruthenium-based metathesis catalysts represent significant advancements in controlling olefin geometry during organic synthesis. Z-selective catalysts, such as the cyclometalated ruthenium complexes Hoveyda Grubbs Catalyst® M2001 and Hoveyda Grubbs Catalyst® M2002, preferentially form Z-alkenes (cis-olefins) from terminal olefines with high stereoselectivity. Complementary to the M2000 series of catalysts, are Umicore’s line of stereoretentive catalysts: Hoveyda Grubbs Catalyst® M2102 and Grubbs Catalyst® M3002. Supported by dithiolate ligands, these catalysts show a broad functional group tolerance and preserve the stereochemistry of the starting olefin in the final product.1
Applications
Pharmaceutical Peptides - Synthesis and Stapling: Z-selective cross metathesis has been employed to synthesize important classes of peptides where the amino acid side chain and the olefin's identity profoundly influence conversion and Z-selectivity.2 The principles of Z-selective metathesis have also been applied to peptides forming parallel β-sheets and for stapling α-helical peptides, a modification imparting beneficial stability and biological activity.2
Natural Products and Pharmaceutical Ingredients: It is anticipated that Z-selective metathesis methods will find extensive industrial use in the synthesis of natural products and pharmaceutical compounds, where precise molecular architecture is crucial for biological activity.3 Z-selective olefin cross metathesis is particularly promising, offering an outstanding methodology for creating new cis carbon-carbon bonds.4 Stereoretentive metathesis is applicable to the production of API’s where E or Z stereochemistry is critical to their activity.5
Fragrances: Z-selective metathesis is valuable for the synthesis of geometrically-defined fragrance molecules such as olfactory macrocycles.3 A notable application is the highly efficient synthesis of Z-macrocyclic musks using stereoretentive ruthenium-based metathesis catalysts.5
Advanced Optical Materials: PPVs (Poly(p-phenylene vinylene) copolymers) with precise stereochemistry are being explored for applications in nonlinear optical spectroscopy for quantum sensing and light harvesting in biological contexts.6
Mechanisms of Selectivity
Z-Selective Catalysts
The Z-selectivity in cyclometalated ruthenium catalysts originates from the olefin approaching the metal center in a side-bound position, cis to the N-heterocyclic carbene (NHC) ligand and trans to the chelating group. This approach is favored due to a combination of steric and electronic effects imparted by the NHC ligand, leading to a preferred syn-metallacyclobutane intermediate that ultimately affords the Z-alkene product..7,8 While traditional olefin metathesis catalysts typically favor the formation of the more thermodynamically stable E or trans isomer, these specialized catalysts, featuring stereogenic metal centers and bulky ligands, overcome the thermodynamic preference for E-alkenes by creating a sterically controlled reaction pathway.7

Stereoretentive Catalysts
Stereoretentive catalysts also operate through a proposed side-bound metallacyclobutane intermediate. However, in this model selectivity arises from the alpha-substituents of the metallacyclobutane positioning themselves away from the large N-aryl groups of the NHC ligand. When a Z-olefin reacts, its beta-substituent points downward in the favored intermediate, leading to the formation of a Z-product upon cycloreversion. Conversely, E-olefinic starting materials result in E-products.1

Summary
Both Z-selective and stereoretentive ruthenium catalysts offer powerful tools for precise control over olefin stereochemistry. Z-selective catalysts achieve high Z-selectivity through a favored reaction pathway, while stereoretentive catalysts preserve starting material geometry. Their utility has been demonstrated in the synthesis of complex targets, including bioactive macrocycles and structurally constrained peptides, highlighting their importance and wide-ranging utility in modern synthetic chemistry.
1. Grandner, J. M.; Shao, H.; Grubbs, R. H.; Liu, P.; Houk, K. N. Origins of the Stereoretentive Mechanism of Olefin Metathesis with Ru-Dithiolate Catalysts. The Journal of Organic Chemistry 2017, 82 (19), 10595–10600. https://doi.org/10.1021/acs.joc.7b02129.
2. Mangold, S. L.; O’Leary, D. J.; Grubbs, R. H. Z-Selective Olefin Metathesis on Peptides: Investigation of Side-Chain Influence, Preorganization, and Guidelines in Substrate Selection. Journal of the American Chemical Society 2014, 136 (35), 12469–12478. https://doi.org/10.1021/ja507166g.
3. Marx, V. M.; Herbert, M. B.; Keitz, B. K.; Grubbs, R. H. Stereoselective Access to Z and E Macrocycles by Ruthenium-Catalyzed Z-Selective Ring-Closing Metathesis and Ethenolysis. Journal of the American Chemical Society 2012, 135 (1), 94–97. https://doi.org/10.1021/ja311241q.
4. US20140106960A1 California Institute Of Technology, Z-selective olefin metathesis catalysts and their synthetic procedure
5. Ahmed, T. S.; Grubbs, R. H. A Highly Efficient Synthesis of Z ‐Macrocycles Using Stereoretentive, Ruthenium‐Based Metathesis Catalysts. Angewandte Chemie International Edition 2017, 56 (37), 11213–11216. https://doi.org/10.1002/anie.201704670.
6. Mandal, H.; Ogunyemi, O. J.; Nicholson, J. L.; Orr, M. E.; Lalisse, R. F.; Ángel Rentería-Gómez; Gogoi, A. R.; Gutierrez, O.; Michaudel, Q.; Goodson, T. Linear and Nonlinear Optical Properties of All-Cis and All-Trans Poly(P-Phenylenevinylene). The Journal of Physical Chemistry C 2024, 128 (6), 2518–2528. https://doi.org/10.1021/acs.jpcc.3c07082.
7. Liu, P.; Xu, X.; Dong, X.; Keitz, B. K.; Herbert, M. B.; Grubbs, R. H.; Houk, K. N. Z-Selectivity in Olefin Metathesis with Chelated Ru Catalysts: Computational Studies of Mechanism and Selectivity. Journal of the American Chemical Society 2012, 134 (3), 1464–1467. https://doi.org/10.1021/ja2108728.
8. Nelson, J. W.; Grundy, L. M.; Dang, Y.; Wang, Z.-X.; Wang, X. Mechanism of Z-Selective Olefin Metathesis Catalyzed by a Ruthenium Monothiolate Carbene Complex: A DFT Study. Organometallics 2014, 33 (16), 4290–4294. https://doi.org/10.1021/om500612r.