Metathesis with PMC
Metathesis: the key features
Due to its versatility, metathesis can be used to synthesize a range of useful products across multiple markets.
The key features of metathesis include:
Functional group tolerance: Modern active pharmaceutical ingredients are among the most complex molecules synthesized on commercial scale. Ruthenium-based metathesis catalysts succeed in forming key bonds in even the most challenging substrates, such as the macrocyclic peptide core of HCV NS3 protease inhibitors like ciluprevir, simeprevir, and related compounds used to treat hepatitis C infections.
Stereoselectivity: Recent advances in ruthenium-based metathesis catalysts offer opportunities for stereoselective metathesis reactions. In particular, Z-selective and stereoretentive catalysts offer new routes for chemists to control the E:Z ratio of newly formed alkenes. While this feature is potentially useful in almost any application, it is particularly advantageous to the production of insect pheromones, which must be prepared in the correct ratio of olefin isomers to be effective as natural pest deterrents for crop protection.
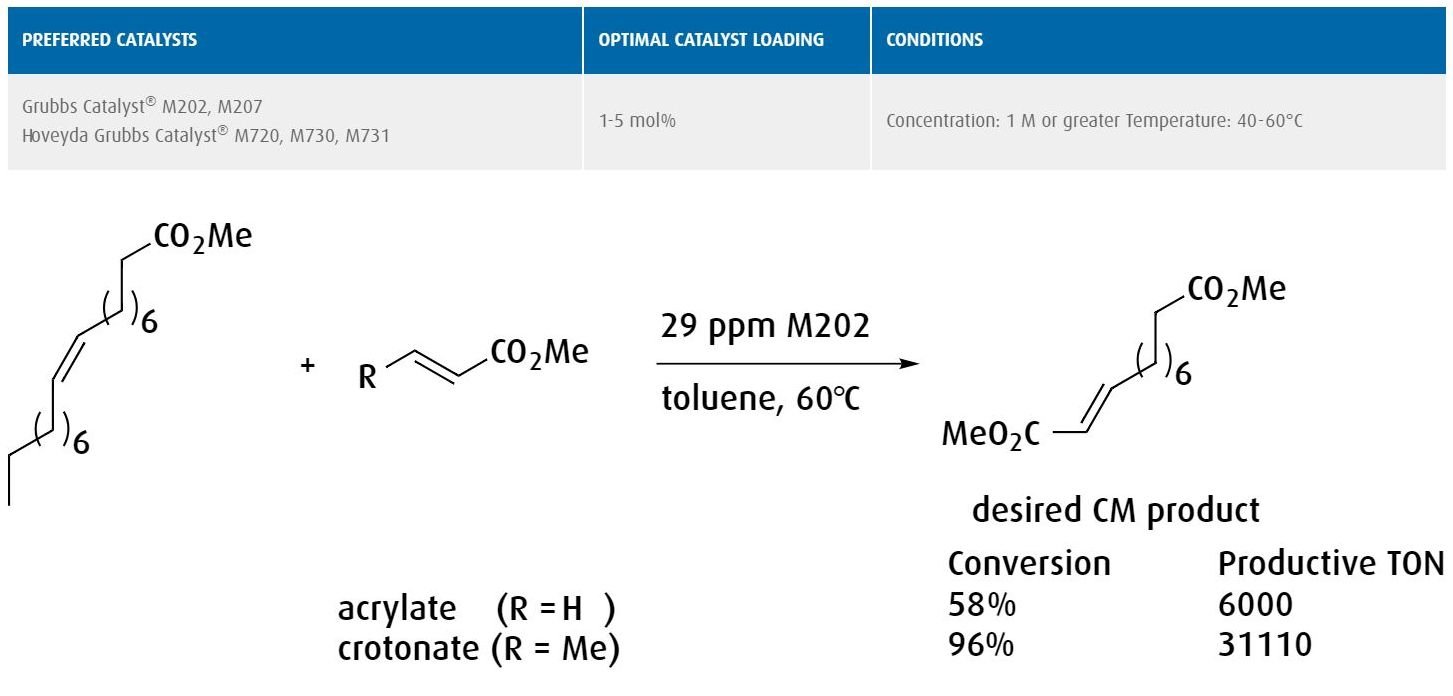
Activity and catalyst longevity: For chemical applications driven by aggressive cost targets, ruthenium-based metathesis catalysts have demonstrated unparalleled activity under rugged conditions. Renewable feedstocks such as soybean oil can be converted into specialty chemical products with turnover numbers in the hundreds of thousands. Thus, even in high-volume, low-margin products, metathesis can deliver significant cost advantages.

Choose your Application
With many versatile reactions to choose from, identifying the right catalyst for your reaction also relies on identifying the right metathesis reaction. Be it ring-closing metathesis, which can be used to synthesize complex polycyclic molecules, or cross metathesis, involving the intermolecular reaction of two unconnected alkenes, we have the on-hand expertise to discuss the best routes to your product, ensuring project success.
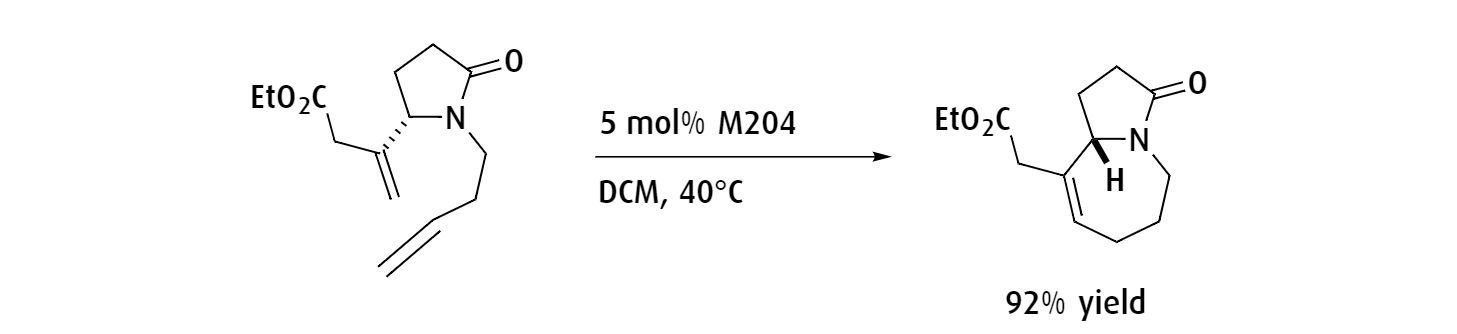
Ring-closing metathesis
Ring-closing metathesis is a common metathesis reaction for any scientist needing to synthesize mid and macro-sized rings, as well as offering an efficient route to synthesize strained or sterically hindered rings. Proceeding via an intermolecular process, this reaction can be made almost irreversible following appropriate reaction optimization.
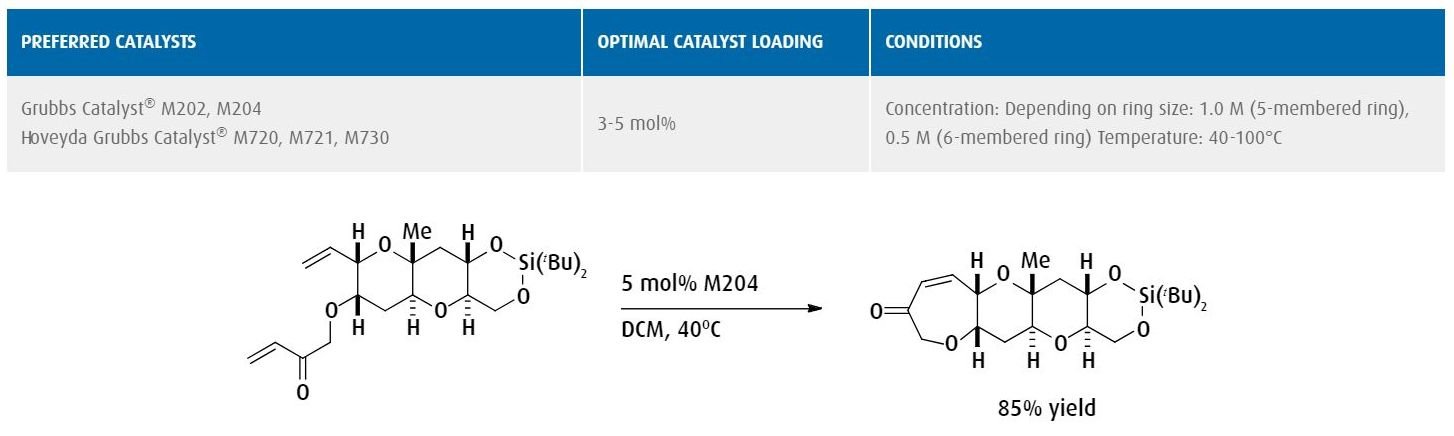 Ring-closing metathesis to form an oxepane ring embedded in (-)-gambieric acid with applications in the pharmaceutical industry.
Ring-closing metathesis to form an oxepane ring embedded in (-)-gambieric acid with applications in the pharmaceutical industry.
Ref: Organic Letters 2015, 17, 4694
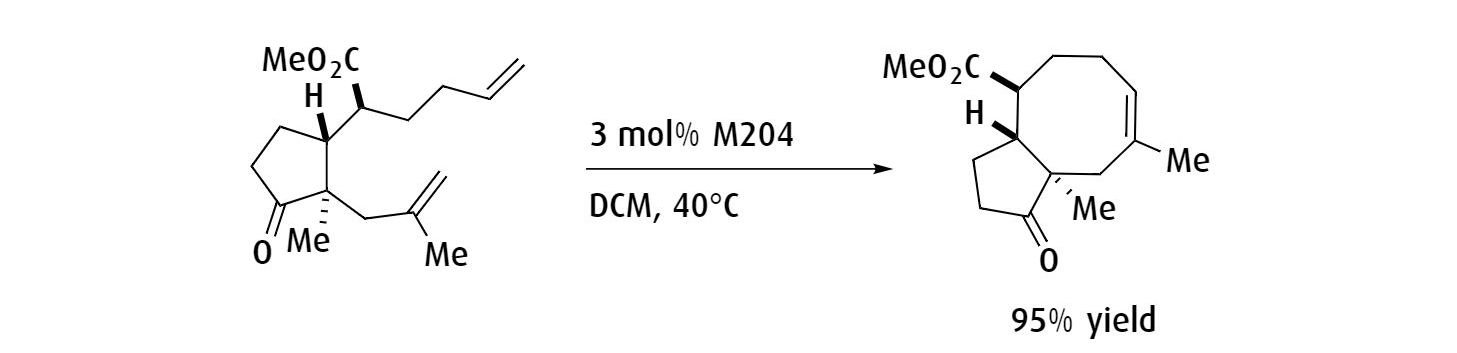
Ring-closing metathesis to yield the synthesis of an 8-membered ring structure of serpendione.
Ref: Tetrahedron Letters, 2005, 46, 1149
Ring-closing metathesis in the synthesis of (-)-stemoamide, a root extract used in Chinese and Japanese folk medicine.
Ref: Journal of Organic Chemistry, 2007, 72, 4246
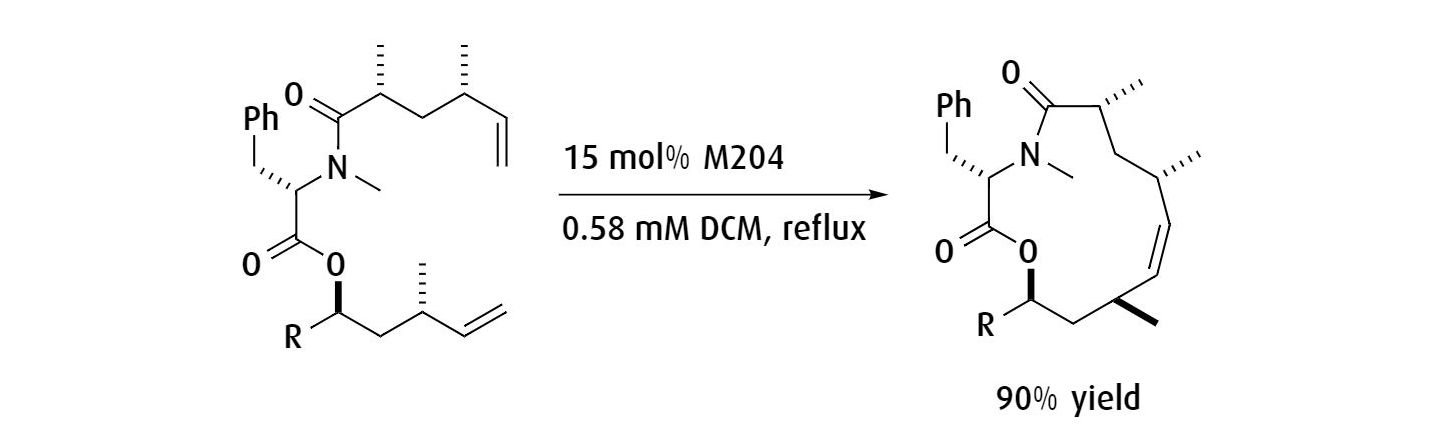
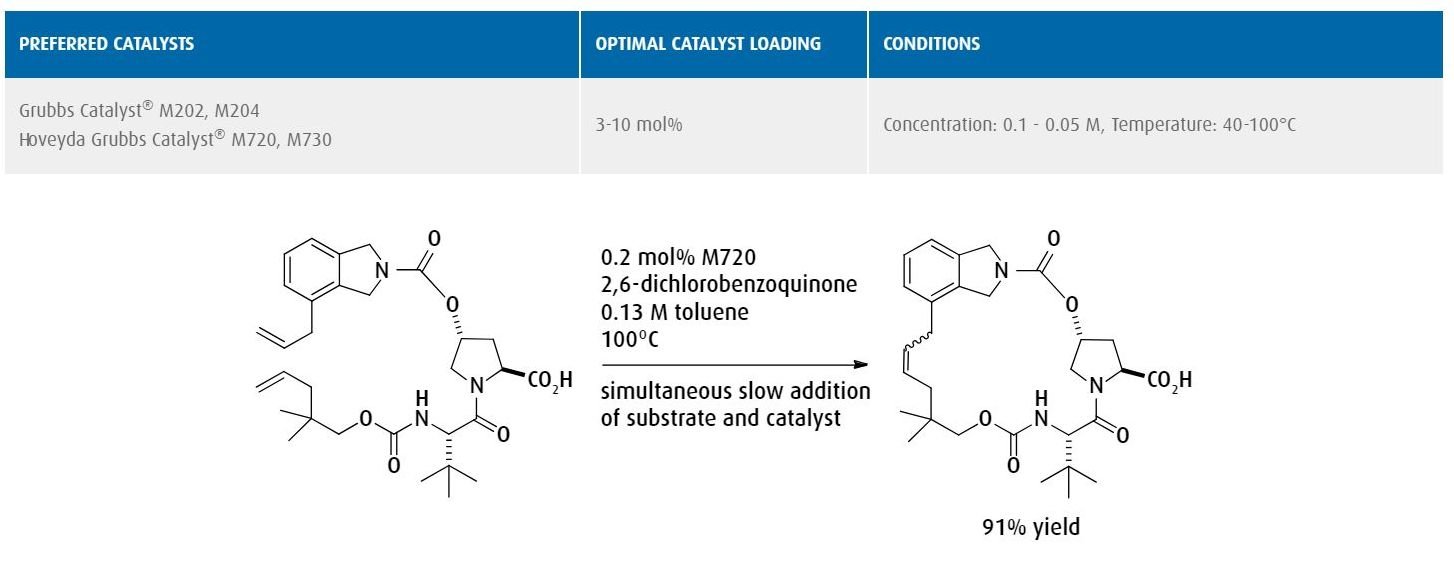 Ring-closing metathesis to form a 20-membered macrocycle used as a protease inhibitor in the pharmaceutical industry.
Ring-closing metathesis to form a 20-membered macrocycle used as a protease inhibitor in the pharmaceutical industry.
Ref: Journal of Organic Chemistry, 2012, 77, 3820
 Formation of a key intermediate in the preparation of the cytotoxic marine natural product (-)-spongidepsin.
Formation of a key intermediate in the preparation of the cytotoxic marine natural product (-)-spongidepsin.
Ref: Organic Letters, 2010, 12, 4392
Formation of a trisubstituted alkene scaffold used for SAR exploration.
Ref: Bioorganic Medicinal Chemistry, 2013, 21, 5707
Cross metathesis
Bringing together two unconnected alkenes in an intermolecular reaction, cross metathesis is an extremely useful reaction that can result in the efficient synthesis of complex and long carbon-carbon chains.
Biscarbocylic acid formation used as precursors to multiple fine chemical products.
Ref: ChemSusChem, 2014, 8, 1143
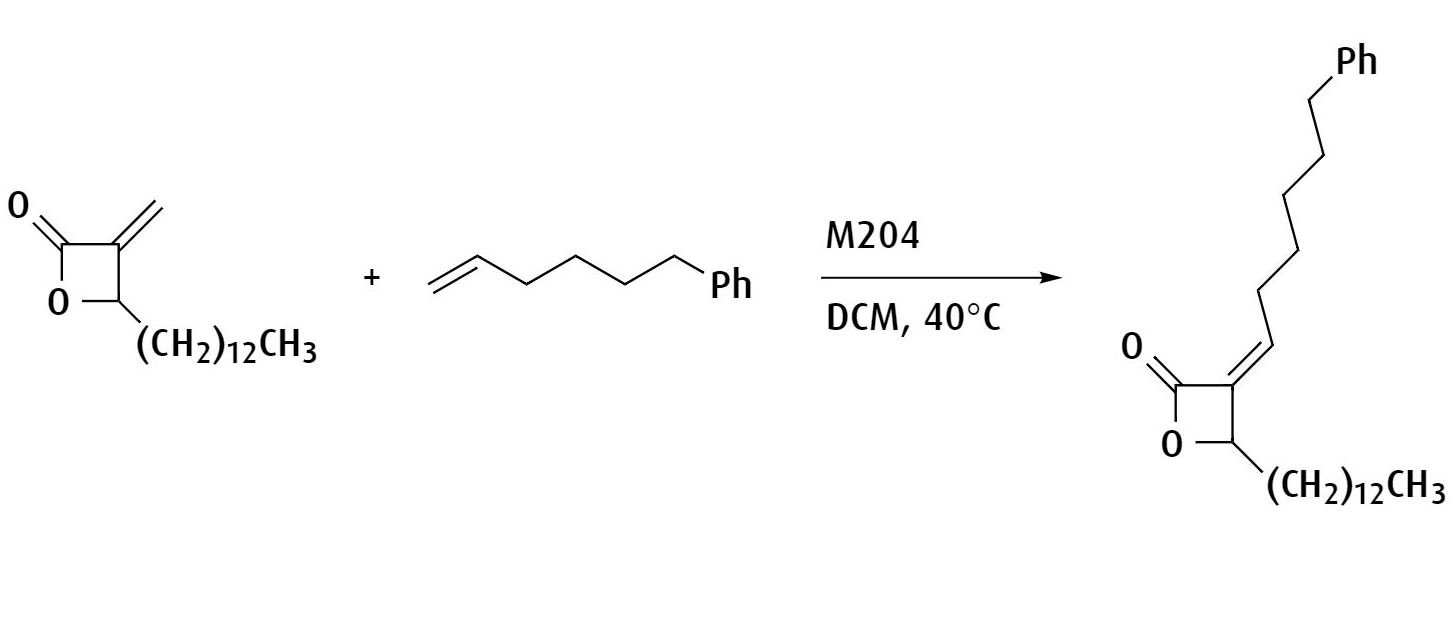 Synthesis of ß-lactone structures bearing a variety of alkyl chains at the 3-position.
Synthesis of ß-lactone structures bearing a variety of alkyl chains at the 3-position.
Ref: Bioorganic & Medicinal Chemistry Letters, 2015, 25, 317
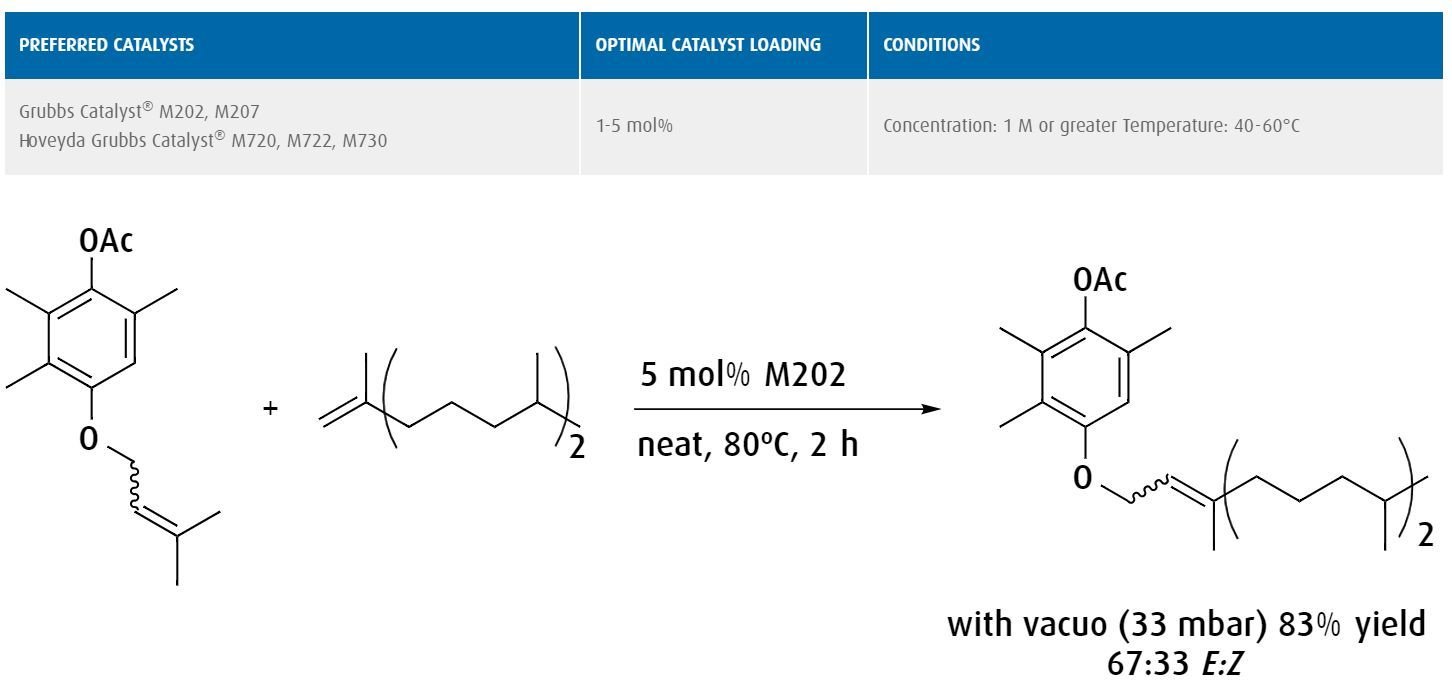
The preparation of vitamin E intermediates by cross metathesis of trisubstituted and disubstituted alkenes.
Ref: Helvetica Chimica Acta, 2006, 89, 797

General reaction procedure
Metathesis reactions are versatile reactions that can be performed in most situations without the need for extensive optimization. But in those difficult synthetic situations, our team of industrial experts will tailor each reaction to the specific requirements for the project, ensuring precise and selective product formation.
There are many factors that must be considered when devising any reaction, including those from catalyst loadings, reaction conditions and the functionality of the product.
Optimizing the catalyst
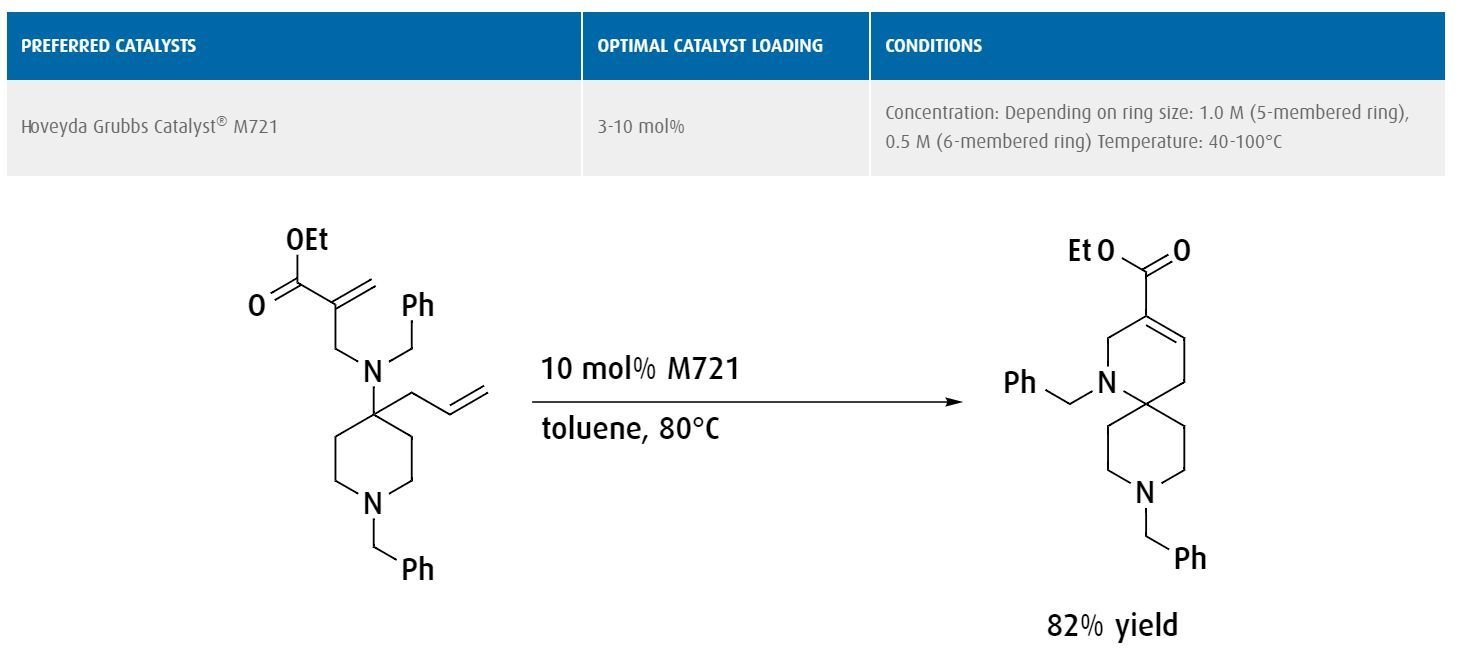
For general metathesis reactions, Hoveyda Grubbs Catalyst® M720 and the Hoveyda Grubbs Catalyst® M730 series are recommended. These catalysts initiate at room temperature and are highly stable, enabling simple storage and handling.
In metathesis reactions involving sterically hindered alkenes, it may be necessary to use a more specialized catalyst. For instance, the Hoveyda Grubbs Catalyst® M721 catalyst can be used to perform sterically hindered ring rearrangement reactions owing to the decreased steric bulk of the protruding ligands.
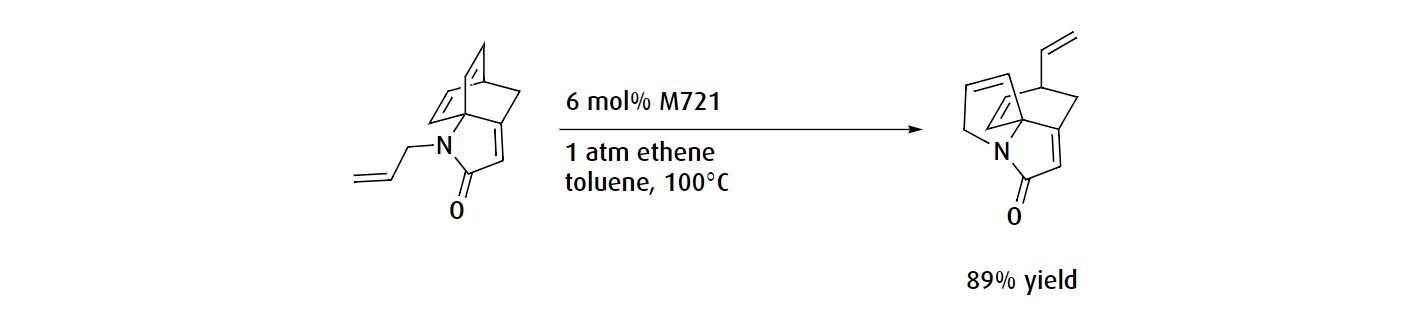 Alternatively, if your substituents are bulky, substituted alkenes, the Hoveyda Grubbs Catalyst® M722 catalyst could result in a higher yield.
Alternatively, if your substituents are bulky, substituted alkenes, the Hoveyda Grubbs Catalyst® M722 catalyst could result in a higher yield.
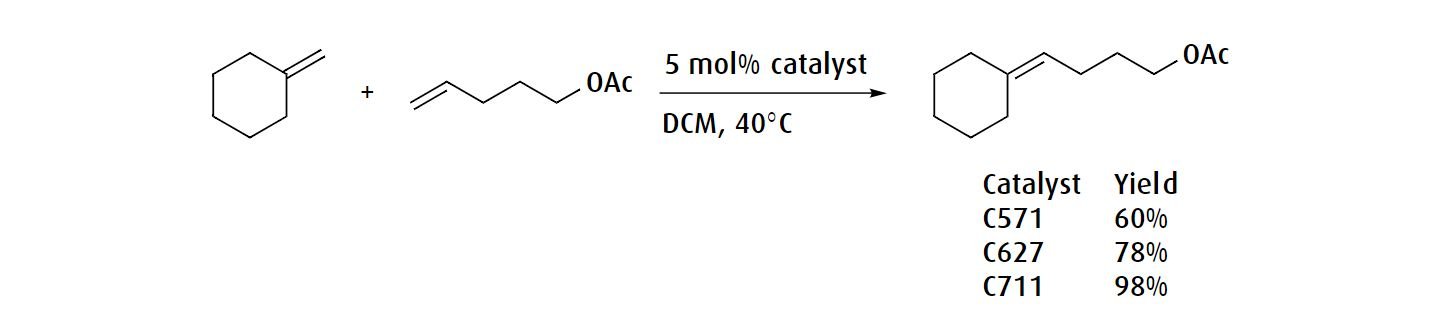
If the reaction conditions are prone to ethylene generation, a catalyst bearing cyclic alkyl amino carbene (CAAC) ligand such as M1001 or M1002 might be more suitable. Additionally, these catalysts can give different selectivity for oligomerization versus ring closure in macrocyclization reactions.
Choosing the right conditions
Choosing the right operating conditions for your industrial reaction relies on careful optimization. Finding the right temperature is vital for catalyst initiation, with low temperatures being advantageous from an environmental perspective. Umicore catalysts typically initiate at low temperatures, between room temperature and 40°C. Furthermore, the exact concentrations of reagents vary according to the specific metathesis reaction: cross metathesis requires concentrated solutions, macrocyclizations require dilute solutions and other ring-closing metathesis reactions require intermediate concentrations.
Likewise, choosing the appropriate solvent to mediate metathesis reactions relies on understanding the various properties of the solvents. Preferred solvents include non-polar, hydrocarbon-based solvents, chlorinated solvents and peroxide-resistant ethers, due to their weak binding affinity to the catalyst complex.
Finally, ensuring the catalytic reaction is devoid of catalyst poisons is also vital. Peroxides oxidize the metal-carbene bond, rendering the catalyst inactive. Furthermore, ethene should also be removed as its presence could also result in catalyst decomposition. In addition, all of the Hoveyda Grubbs Catalyst® products are air and moisture stable as solids, but in solution are vulnerable to oxygen. Therefore, reactions need to be run in the presence of argon or nitrogen gases to ensure the exclusion of oxygen.
Optimizing the reaction procedure
Beyond conditions, there are multiple aspects of the synthetic procedure that must be considered for the successful completion of any reaction. Strongly coordinating functional groups must be masked so as to not disrupt the catalyst activity; the concentration of the various substrates must be optimized to prevent substrate polymerization, but encourage the desired format of metathesis.
Furthermore, to prevent unwanted side-effects of metathesis reactions such as the isomerization of alkenes, it is sometimes necessary to use additives to suppress the unwanted reactions. For instance, mild acids such as acetic acid can be added to reactions to prevent hydride formation, as illustrated in the following diagram:
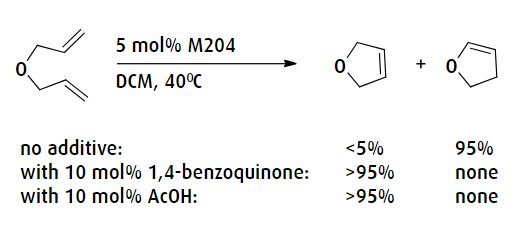
Metathesis reactions that bring together two terminal alkenes produce ethene as a by-product. Although ethene is a gas, it is soluble in organic solvents and can remain in the reaction mixture. Ensuring that ethene or any other gaseous by-product is efficiently removed will drive the reaction equilibrium toward completion. This can be accomplished by bubbling an inert gas through the reaction mixture over the course of the reaction. On scale, this technique is used frequently to help maximize catalyst lifetime.
For the selective formation of cis- or Z-alkenes, one can use the Hoveyda Grubbs Catalyst® M2001 and Hoveyda Grubbs Catalyst® M2002, which provide selectivity in both ring-closing or cross metathesis reactions.
Supportive Services
As leading experts in catalyst research and development, Umicore has decades of experience developing ground-breaking chemistry. We offer a range of supplementary services to help scale your chemical reaction, regardless of your reactants, products and market. We work across your development pipeline to devise strategies to deliver solutions to solve any optimization challenges faced along the process; from research to product launch and beyond.
Let’s work together to accelerate your product synthesis.
We will work with you to define the best route to your synthetic target or optimize the production to maximize yield or selectivity. This could be through identifying new reaction routes by devising alternative retrosynthesis, which are then screened as potential reactions at all scales of the industry.
Understanding the best route to your product comes down to understanding the complex chemistry driving your reaction. We work across all phases of a project’s pipeline, from the initial investigations into the viability of catalytic reactions, to optimizing processes for large-scale production. Our understanding and experience in the manufacturing and development process means we know what expertise needs to be deployed at each stage of a project.
At Umicore, we have the expertise in global manufacture and development to ensure the successful and reliable supply of reagents, as well as the technical expertise to identify the best catalyst and conditions for your reaction. Our solutions are tailored to each of our customer’s reactions and each catalyst loading is optimized to the requirements of each project.