Executive Summary: YPhos ligands
Phosphine ligands are a well-regarded and versatile class of ligand, commonly employed in cross-coupling catalysis. Due to their ability to help chemists forge much-sought-after carbon-carbon bonds across a wide range of industries. Typically possessing the general formula, PR3, the substituents attached to the phosphine atom are highly variable, allowing scientists to intricately tune phosphine ligand catalysts to fit specific needs based on the reaction.
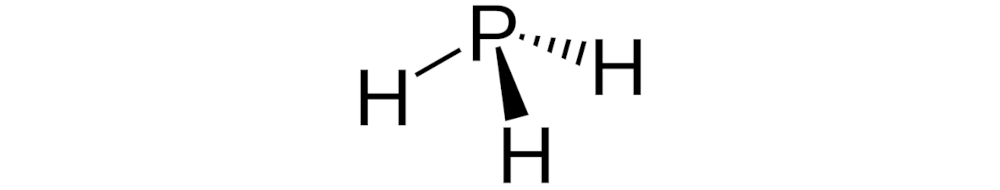
Figure 1 - The structure of phosphine.
Despite these favourable qualities, however, the boundaries of activity and specificity can always be pushed.
This is the work that is being done by a team led by Viktoria Gessner, chair of Inorganic Chemistry and Ruhr University Bochum, to create a new class of cutting-edge phosphines – known as YPhos ligands.
In this blog, we will explore the history and hallmarks of this innovative technology and delve into the practical benefits they provide to chemists.
What are YPhos ligands?
While phosphine ligands typically have an aryl or alkyl substituent, YPhos ligands have an ylide in its place, consisting of a carbon atom with a formally negative charge – a carbanion – attached to the phosphorous centre of the ligand. This electron-rich carbanion creates a highly negatively charged environment and grants uniquely high donor properties compared to the more conventional alkylphosphines available on the market.
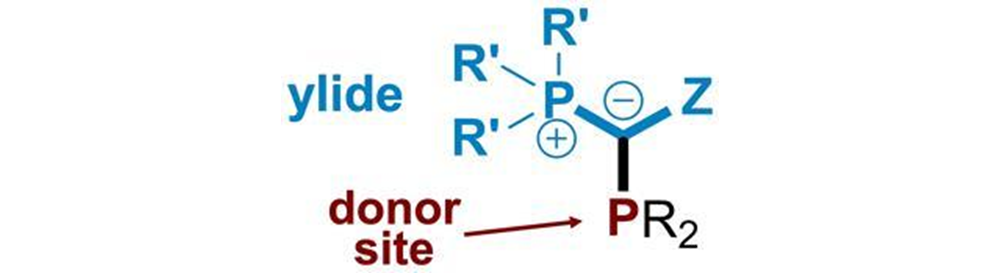
Figure 2 - The general structure of a YPhos ligand. Where you would normally expect
an aryl or alkyl subtituent, the phosphine ligand as a ylide group (coloured blue).
These electron rich ligands show superior activity, allowing for more effective reactions under milder conditions. This, of course, is particularly pertinent to process chemists who will always be on the lookout for more efficient reactions, in terms of both yield and energy expenditure.
The addition of the ylide substituents is done through three main routes, all of which start with the cheap and readily available phosphonium salts.
- A double deprotonation with a metal based, followed by reaction with a halophosphine.
- Reacting the corresponding ylid with half an equivalent of dialkylhalophosphine
- Reacting the corresponding ylid with a dialkylhalophosphine, followed by a deprotonation of the resulting alpha-phosphino substituted phosphonium salt with a base.
These preparation reactions differ in terms of condition and reagent requirements, allowing chemists the choice of the most convenient route for them.
The ligands have also been specifically designed for high performance, with their intricate structure playing a role in their efficiency.
Why YPhos?
YPhos ligands excel when they are used within palladium and gold catalysis.
Generally, they are applied to cross-coupling reactions in both C-C and C-N bond-forming reaction. These bonds are normally challenging to form and fundamental to a wide range of chemicals across both the agrochemical and fine chemical industries. Within both of these bond-forming reactions, the monoligated palladium YPhos complex specifically demonstrates higher activity.
With higher activity and lesser energy requirements, processes can also be made more sustainable, providing chemists with greener synthesis pathways.
Meanwhile, gold YPhos complexes have shown higher activities in hydroamination reactions and in more specialised C-H and C-C bond forming reactions.
It was this industrial relevance which initially drove Professor Gessner’s team towards these ligands. Upon noticing the unique electron-donating ability of them and tuning their catalytic activities by adjusting substituents, the team pivoted towards monoligated palladium catalysts due to the potential industrial uses.
Now, Professor Gessner’s team has designed a range of ligands, each for specific reaction uses and are always innovating to expand the versatility of the technology.
Umicore offers its customers globally a selection of YPhos ligand and catalyst systems on both research and commercial scales. To discover how YPhos technology can serve your specific needs, find our more information about the acquiry here. As Professor Gessner said in her recent Chemistry World interview, ‘Frankly, if one is interested in coupling reactions, I would say you should always try the YPhos ligands.’