Executive summary: Cross-Coupling
Due to the importance of carbon-carbon bonds in organic synthesis, intuitive chemistries to form these bonds are at the heart of the chemical industry. Cross-coupling reactions represents an intuitive solution to synthesise these carbon-carbon bonds. For decades, Umicore has explored and aided the advancement of this chemistry from its foundations and possess key expertise in the different reagents used and how they provide chemists with a sustainable and reliable way to produce the much-coveted carbon-carbon bond.
The foundations of life
Carbon-carbon bonds are fundamental to life on Earth, making up the chemical skeleton of nature itself, as well as most organic molecules. The bond, renowned for its stability, is both highly sought after by process chemists and complex to create.
To overcome this challenge, cross-coupling is widely employed and makes up some of the most commonly used carbon-carbon bond formation reactions. As our Business Development Manager, Philip Wheeler, discusses in this Chemistry World article, the chemistry has seen a meteoric rise to prominence over the late 20th and early 21st century, finally receiving international recognition in 2010 when Richard Heck, Akira Suzuki and Ei-ichi Negishi were jointly awarded the Nobel Prize for their respective work in the development of palladium-catalysed cross-coupling reactions and their role in developing it as a versatile chemical tool.
Cross-coupling is commonly applied in pharmaceutical development, as well as when synthesising API and hAPI solutions. Cross-coupling solutions are also used for sustainable chemical syntheses across the agrochemical, electronic and fine chemical industries. Its ability to construct carbon-carbon bonds has a reach outside of chemistry too, providing all fields that involve organic molecules, including both biology and materials science, with a handy tool to do so with.
At Umicore, we offer a wide selection of highly active precatalysts and catalysts that provide users with highly efficient cross-coupling reactions. Our partners also gain access to our outstanding proprietary, patent-protected technologies and unrivalled service offerings to ensure they get the most out of their offerings. Additionally, through collaborative partnerships, Umicore has recently driven forward its offerings of this technology, as discussed by our Director of Global Business Homogeneous Catalysts, Volker Raab in this Chemistry World article.
How does it work?
Cross-coupling reactions work off the basis of reductive elimination, leaving a newly formed carbon-carbon bond. The reacting partners are a formal nucleophile, activated with a carbon-metal bond, and a formal electrophile, activated with a carbon-(pseudo)halogen bond. The reaction as a whole is promoted by a base and proceeds via a catalytic cycle, with the mechanism consisting of three distinct steps: oxidative addition, transmetalation and reductive elimination.
An array of different cross-coupling reactions exist, with most being developed for the coupling of two specific functional groups. For example, the Suzuki reaction couples boronic acid derivatives to organohalides, whereas Sonogashira coupling joins terminal alkynes and aryl or vinyl halides.
Some coupling reactions are classed as such due to the similarity in reaction pathway, such as the Buchwald-Hartwig amination which involves the formation of carbon-heteroatom bonds instead of the conventional carbon-carbon bond.
While several different metals can be catalysts for cross-coupling reactions, palladium-based catalysts are currently the most predominantly used. The catalysis is also usually homogeneous (where the reaction takes place in the same phase as the catalyst), and while there is some recent research into heterogeneous cross-coupling catalysis, currently it offers poor selectivity and is not widely used.
The active catalyst is in a Pd(0) form, being promoted to Pd(II) when it forms the catalytic intermediates and reforming the original Pd(0) catalyst after the reductive elimination step. Unfortunately, the Pd(0) catalyst can be unstable when exposed to air, making transporting, storing and handling of it tricky. To combat this, the palladium catalysts are usually provided to users in the form of a ‘precatalyst’ – a stable precursor that can be easily activated to create the desired species in situ.
A common strategy employed is to combine a stable Pd(II) salt with the ligand of choice under a set of conditions that then creates the desired active Pd(0) catalyst. The steps involved in this must be robust and easily reproducible, as not to introduce additional complications for process chemists during scale-up, such as the introduction of unwanted side products.

Figure 1 – The general mechanism for cross-coupling reactions
Buchwald precatalysts
The Buchwald palladacycle precatalyst family were developed by Stephen Buchwald and his team at the Massachusetts Institute of Technology. Alongside these, Buchwald also co-developed the Buchwald-Hartwig amination reaction and a class of biaryl phosphine ligands, known as Buchwald ligands, that grant higher activity with their electron richness and sterics – although Buchwald precatalysts can be used with almost any cross-coupling reaction and supporting phosphine ligand.
Buchwald precatalysts grant several advantages over conventional pallium precatalysts. Firstly, the Buchwald precatalyst is reliably converted to the catalyst form under treatment of mild base. It also comes with the desired ligand attached in a 1:1 ratio, limiting the variability of the provided complex.
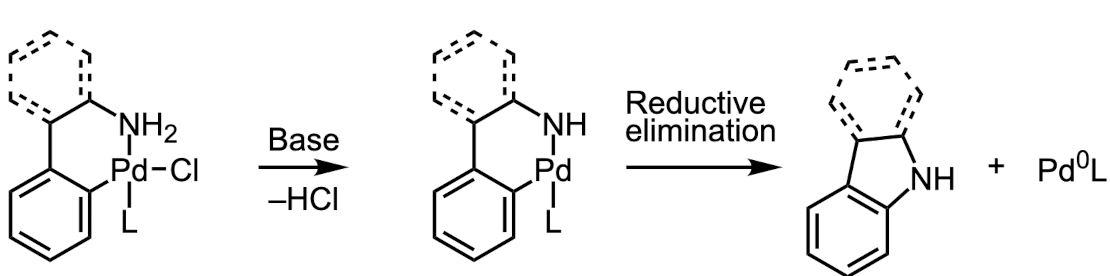
Figure 2 – The mechanism of the Buchwald palladacycle activation
The 1:1 ratio also promotes the monoligated palladium catalyst, which undergoes the initial oxidative addition reaction faster than its PdL2 counterpart.
Umicore offers a comprehensive list of high-purity Buchwald precatalysts for all uses, from research to commercial.
Hazari precatalysts
A recent addition to the PMC portfolio is the Hazari precatalyst, developed by Nilay Hazari’s team at Yale University. The air- and moisture-stable compound is uniquely compatible with a broad range of ligands, meaning it possesses greater substrate compatibility.
Umicore also offers an unligated dimeric version of the Hazari precatalyst that can be readily ligated by the user in situ to screen for and identify the most efficient metal-ligand complex.
The future of cross-coupling
The invention of new catalysts, such as Hazari and Buchwald discussed above aptly demonstrate that despite being discovered almost 50 years ago, cross-coupling remains a fertile area of research with exciting new advances still being made. For example, much modern research is beginning to focusing on the development of non-traditional substrates, thus broadening what this already-versatile chemistry can do. Furthermore, while aryl (attached to an aromatic ring) and alkenyl (attached to a C-C double bond) sp2 carbon centres can be joined predictably, alkyl sp3 centres are more challenging and a robust method, tolerant of functional group variation continues to elude researchers.
Modern, high-throughput experimentation is spurring cross-coupling research forward. As new discoveries are made, this innovative chemistry will undoubtedly lead to a more sustainable future, providing chemists with increased efficiency and improved reaction pathways, as well as more precise tools to create chemicals beneficial to us all.
To explore our cross-coupling offerings, follow the link to our webpage here: https://pmc.umicore.com/en/products/technologies/cross-coupling-reactions-with-arenes/