Executive Summary: Alkene Metathesis
Alkene metathesis is an innovative catalytic reaction that provides a versatile method to build molecules through carbon-carbon bond formation. In this blog post, we’ll discuss the importance of this cutting-edge technology, its history and how metathesis catalysts are driving innovation in the pharmaceutical and fine chemical industry.
Better ways to bond
Carbon–carbon bonds are among the most important bonds found in nature. Their stability, combined with the ability to form into long chains, makes them the ideal bricks for building new chemical compounds.
As such, it should be of no surprise that for any organic chemist, sustainable and intuitive tools for developing carbon-carbon bonds are of high priority. Most of the methods available involve pre-activation of one or both of the carbon atoms (for example, an alkylation reaction requires a metal enolate and an alkyl halide). However, for industry, the ideal tool for forming carbon-carbon bonds would not require any pre-activation or stoichiometric reagents and would proceed in high yield and with predictability.
Many reactions come close to the above criteria, but with alkene metathesis, the proof is in the pudding. Alkenes are cheap, the process is easy and the products are valuable. Consequently, metathesis has been adopted by chemists across wide array of fields, from petrochemicals to pharmaceuticals to advanced materials. It would be difficult to identify a field that uses the tools of organic chemistry that has not also found a use for alkene metathesis.
History of Metathesis
Alkene metathesis started as an oddity. As our Business Development Manager, Philip Wheeler, discusses in his Chemistry World article, propylene’s disproportionation into ethylene and 2-butene was discovered long before anyone knew how it worked or could be applied. Since then, decades of work by both industrial and academic chemists have led to a detailed understanding of this process and its transformative abilities. So much so, that Robert Grubbs, Richard Schrock and Yves Chauvin, would earn the 2005 Nobel prize in Chemistry for the development of the metathesis method in organic synthesis. Today, metathesis catalysts have evolved into an advanced and versatile tool with the ability to be applied to a wide variety of targets for scale-up.
The original metathesis catalysts were of the “first-generation” type, bearing two phosphine ligands. Development of the second-generation catalysts and the Hoveyda–Grubbs modified catalysts were largely spurred by the need for more active catalysts that could affect transformations that the first-generation systems could not, such as the metathesis of sterically demanding and electron-poor alkenes.
While these improved catalysts broadened the realm of alkene metathesis reactions, there are instances where the first-generation catalysts continue to provide excellent or superior results in a given metathesis reaction.
At Umicore, we can provide our customers with full access to proprietary, patent-protected metathesis technologies. By offering access to the widest metathesis portfolio, our customers can develop more efficient and sustainable syntheses, produce more competitively and keep or extend their leading positions in their markets.
How do metathesis reactions work?
Alkene metathesis is a chemical reaction in which two carbon-carbon double bonds (also known as alkenes) come together and exchange with one another, forming new alkeneic products in the process.
In the simple example below, symmetrical disubstituted alkenes (each with blue or red substituents) are converted to non-symmetrical alkenes (with one blue and one red substituent).

Figure 1: Graphic representation of a metathesis reaction
This reaction won’t occur without a catalyst, and since catalysts work in both directions, you’ll usually end up with a thermodynamic mixture of products. In the above example, you’ll end up with 50% of the red/blue product and 25% each of the red/red and blue/blue compounds.
Sometimes, synthetic chemistry does not do what you want. Any organic chemist will tell you that when pressed on the viability of chemical reactions. This can be caused by the favoured submission of side reactions, or by the fact that the reaction in question does not lead to the desired product in a high enough yield.
Of course, alternative routes for the reaction can be devised. One such alternative could involve additional steps that approach the problem from a different angle. However, this workaround could prove to be an unnecessary complication that can easily be avoided. Instead, by using alkene metathesis catalysts, new synthetic routes for substrates can potentially be devised. One example is in the synthesis of multi-functional polycyclic lactams which have potential applications in medicinal chemistry. Here, Grubbs’ catalysts have shortened the number of steps by achieving a hindered synthesis in a high-yielding reaction.
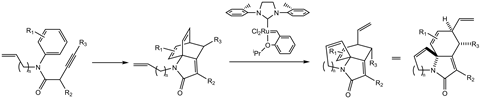
Figure 2: The two-step reaction to form complex polycyclic lactam products.
This illustrates just one example of how a complex polycyclic lactam can be synthesised in a single high-yielding, two-step process, the second of which involves a ring-closing metathesis reaction. Here, robust metathesis catalysts demonstrate their aptitude for achieving fundamentally difficult reactions on strained and complex reagents. Furthermore, the catalyst used is versatile, tolerating various functional groups, including tertiary amines. Where previous synthetic methods had relegated the lactam product to a low concentration side-product, ring-closing metathesis now forms it as the major product.
A greener tool
Because alkene metathesis is promoted by a catalyst, a well-designed process will generate little waste. To learn more about the green benefits of metathesis we asked our Technical Sales & Marketing Manager, Jessica Gomes-Jelonek, for her thoughts.
“I think what makes metathesis so advantageous is everything else it does, in addition to the synthesis. Since there are likely many routes to the target molecule, a chemist will need to consider additional factors that aid them. What makes it so important is everything else it can do: it is green, efficient, selective and high-yielding, and offers significant advantages over other alternative reaction pathways.”
In recent years, the gradual depletion of fossil resources and the strong need to limit the adverse environmental and health impacts of petroleum-based chemicals have pushed driven use of renewable natural resources as green alternatives. In the alkene metathesis field, readily available and inexpensive plant-derived molecules containing carbon–carbon double bonds are currently considered ideal raw materials to be employed for the synthesis of valuable compounds within the chemical industry.
Among all renewable raw materials, unsaturated fatty acid esters from vegetable oils, such as methyl or ethyl oleate, emerge as the most attractive chemical platforms for alkene metathesis. These compounds can be easily transformed into a variety of high-value products via cross-metathesis (CM) with both unfunctionalized and functionalized alkenes.
To get a better understanding of metathesis’ future, there is no one better to ask than one of its pioneering researchers, Professor Robert Grubbs. In our previous conversation with him, he discussed his thoughts for the next frontier of this innovative chemistry.
“Chemistry is impacting research in all kinds of different directions. New technologies, in particular new chemical reaction pathways, being developed are opening up new, exciting avenues in pharmaceutical research. This is providing new pharmaceuticals and new pathways to existing molecules, making them cheaper and easier to come by all while opening up possibilities to treat new diseases. In the long run, this will greatly impact healthcare costs by actually making people better, rather than having to provide long-term treatment.”
To learn more about metathesis technologies, check out our web page: https://pmc.umicore.com/en/products/technologies/metathesis/
And our interactive digital brochure: https://pmc.umicore.com/metathesis-guide/